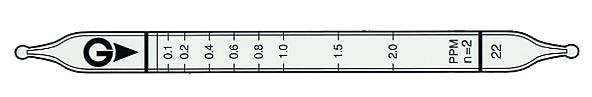
| Measuring Range |
| 0.02 to 0.05 ppm |
0.05 to 2 ppm |
2 to 5 ppm |
|
| Number of Pump Strokes |
|
| Correction Factor |
|
| Sampling Time |
2 minutes per pump stroke |
| Detecting Limit |
0.01 ppm (n=5) |
| Colour Change |
Yellow → Red |
| Reaction Principle |
Diborane reacts with mercuric chloride to produce hydrogen chloride.
HCl react with indicator to discolour to red.
B2H6 + 6HgCl2 → 2B2(HgCl)3 + 6HCl
HCl + Base → chloride |
| Coefficient of Variation |
10% (for 0.05 to 0.4 ppm), 5% (for 0.4 to 2 ppm) |
| Shelf Life |
2 Years |
| Corrections for temperature & humidity |
Temperature correction is necessary |
| Store the tubes at cool and dark place. |