Polytec-II |
NO.25 |
| Qualitative Analysis |
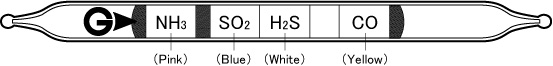 |
Specification
| Number of Pump Stroke | 1 |
| Sampling Time | 1 minute per pump stroke |
| Shelf Life | 2 Years |
| Reaction Principle | NH3:Neutralising reaction SO2:Free acid is produced →acid-base reaction H2S:Sulphuric compound is produced CO:Reduction reaction with Palladium compound |
Colour Change of Each Layer
| Substance | Conc. (ppm) |
Colour Change | |||
| NH3 | SO2 | H2S | CO | ||
| Ammonia | Yellow (Inlet) Yellow (9mm) |
- | - | - | |
| Hydrogen Chloride | - | Yellow (3mm) | - | - | |
| Chlorine | - | Yellow (3mm) | - | - | |
| Sulphur Dioxide | - | Yellow (Inlet) Yellow (6mm) |
- | - | |
| Nitrogen Dioxide | - | Purple (Inlet) | - | - | |
| Hydrogen Sulphide | - | - | Brown (Inlet) | - | |
| Carbon Monoxide | - | - | - | Blackish brown (Inlet) | |
| Hydrogen | - | - | - | Blackish brown(Whole layer) |
|
| Olefin HCs | - | - | - | Blackish brown(Whole layer) | |
| Mercaptans | Blackish brown (Inlet) | ||||
(1) Amines give the same discolouration with Ammonia. |
|||||